Contributed talk
Tuning the closed/open shell character of molecular dimers by their coupling motifs
Jan Patrick Calupitan1,2,*, T. Wang1,2, P. Angulo-Portugal1,2, A. Berdonces-Layunta1,2, D. Casanova2,3, M. Corso1,2, D. G. de Oteyza1,2,3
1 Centro de Fisica de Materiales CFM/MPC, CSIC-UPV/EHU, San Sebastián
2 Donostia International Physics Center, 20018 San Sebastián
3 Ikerbasque, Basque Foundation for Science, 48009 Bilbao
* Present Address: Sorbonne Université, Institut Parisien de Chimie Moléculaire, Paris
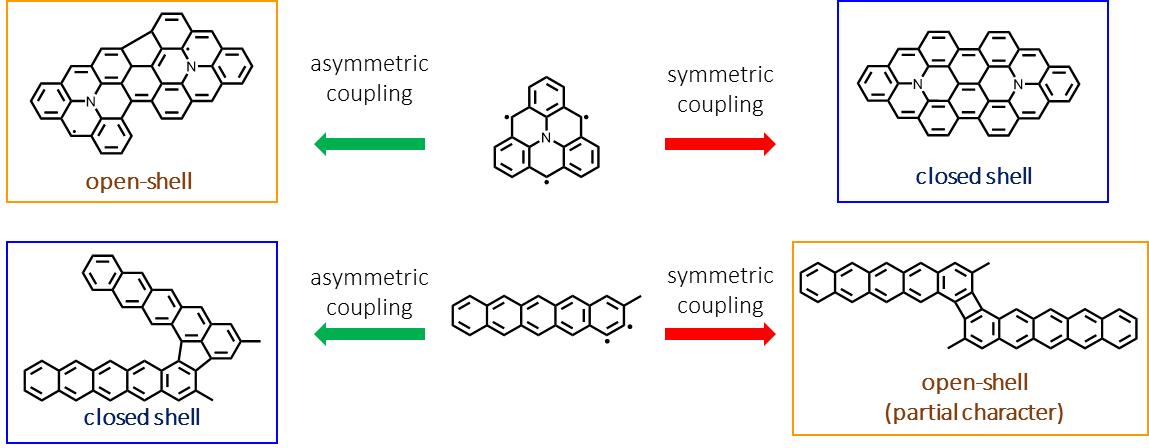
On-surface synthesis (OSS) has enabled the unprecedented fabrication and characterization of a myriad of pi-magnetic molecular materials [1]. Iconic examples include triangulenes, which are magnetic due to sublattice imbalance, and acenes, which have increasing diradical character with size. Recently, we showed that substituting a N atom in the C network of triangulene, to make aza-triangulene, enriches its surface chemistry, leading to opposite charge transfer directions on Au(111) and Ag(111) [2]. In this presentation, we illustrate a new strategy to expand the chemical space of such structures: by forming dimeric derivatives with various coupling motifs. For aza-triangulene, forming a symmetric dimer led to a structure without magnetic fingerprints, while asymmetric coupling motifs led to structures that display Kondo resonance (with associated detection of SOMO/SUMO). Meanwhile, for acenes, we show an opposite trend with pentacene [3], the largest acene widely accepted to have a closed-shell character. A dibrominated sterically hindered derivative was shown to form dimers on Au(111), with varying diradical character according to their coupling motifs. A symmetric dimer, with a four-membered ring as a coupling motif, displayed a low HOMO-LUMO gap typically associated with diradicaloid molecules. Meanwhile, asymmetric dimers with five-membered rings as coupling motifs showed a considerably higher gap despite having the same extent of pi-conjugation. DFT confirmed the diradical character of the symmetric dimer, which we associate to the antiaromaticity of the central cyclobutadiene ring. Understanding these structure–property relations is desirable not only for fundamental reasons but also for designing new complex and functional molecular structures. We illustrate this with DFT results on prospective new molecules, which illustrate further fine-tuning of magnetic properties.
[1] D.G. de Oteyza, T. Frederiksen, J. Phys.: Condens. Matter, 34, 443001 (2022)
[2] T. Wang et al., J. Am. Chem. Soc. 144, 4522-4529 (2022)
[3] T. Wang et al., J. Am. Chem. Soc. 145, 10333-10341 (2023)