Contributed talk
Design and synthesis of open-shell PAHs: Chichibabin derivatives
Saleta Fernández, F. García, M. Vilas-Varela, D. Peña
Centro Singular de Investigación en Química Biolóxica e Materiales Moleculares (CiQUS) and Departamento de Química Orgánica, Universidade de Santiago de Compostela, 15782, Santiago de Compostela (Spain)
Polycyclic aromatic hydrocarbons (PAHs) represent an extensive family of organic compounds form by the fusion of aromatic rings. Open-shell PAHs are a subclass of this family of compounds, characterized by the presence of unpaired electrons [1]. The existence of unpaired electrons in these molecules may introduce fascinating properties, such as two photon absorption enhancement, singlet fission, chiroptical, or amphoteric redox behaviour, which makes them highly attractive for applications in the field of materials science [2,3]. Among the multitude of open-shell PAHs realized to date, Chichibabin’s hydrocarbons (e.g. 1-OS) have been extensively studied due to their characteristic structures and stability under ambient conditions [4].
Based on previous molecular designs (e.g. 2-OS) [5] in this contribution we report our efforts in the design and synthesis of stable Chichibabin derivatives, characterised for presenting a bianthracene unit as the central molecular core and fluorenyl fragments as end-groups, to study their electronic and spin states, and their integration in molecular devices [6].
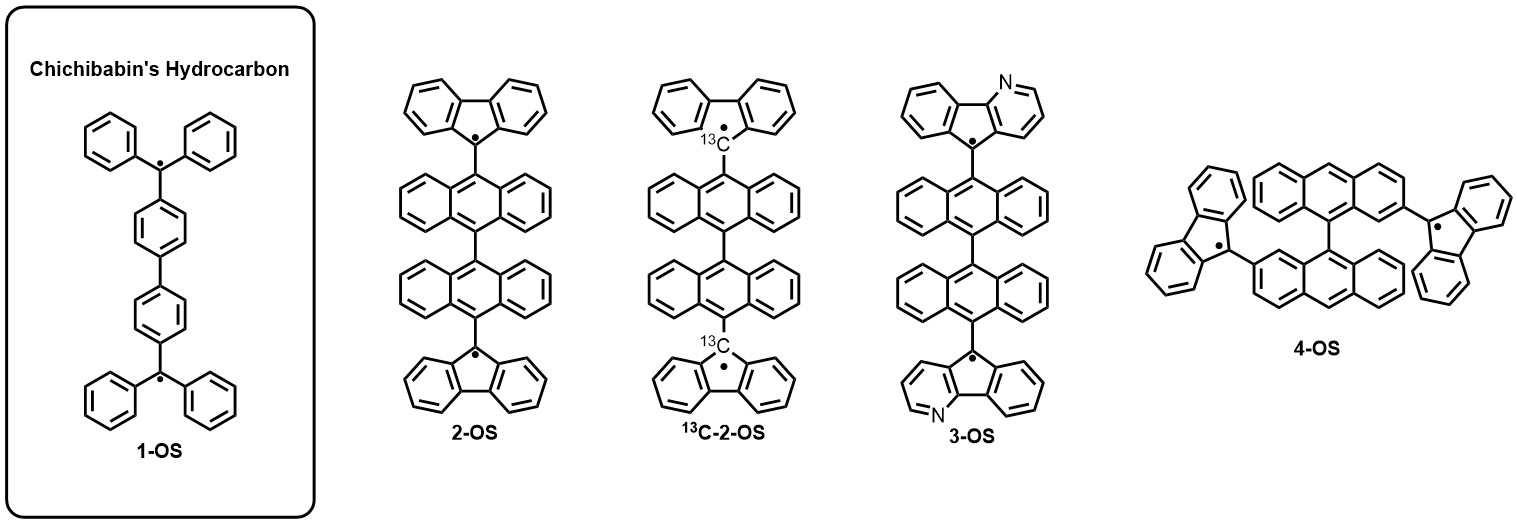
Figure 1: Examples of Chichibabin derivatives synthesized in this contribution.
[1] Z. Zeng et al., Chem. Soc. Rev., 44, 6578 (2015)
[2] Z. Zeng et al., Chem. Soc. Rev., 41, 7857 (2012)
[3] M. Abe, Chem. Rev., 113, 7011 (2013)
[4] A. Tschitschibabin, Chem. Ber., 40, 1810 (1907)
[5] Z. Zeng et al., J. Am. Chem. Soc., 134, 14513 (2012)
[6] T. Y. Baum et al., Nano Lett., 22, 8086 (2022)